Article 1 Accelerate the implementation of innovation-driven development strategies
Support Shenzhen to build artificial intelligence, life information and biomedical laboratories and other major innovation carriers, and explore the construction of an international science and technology information center and a new system of medical sciences. Explore the securitization of intellectual property rights, and establish a standardized and orderly construction of a property rights trading center for intellectual property and scientific/technological achievements.
Article 2 Accelerate the construction of a modern industrial system
Carry out pilot reforms of market access and regulatory systems and mechanisms, establish a more flexible and prudential and inclusive regulatory system, actively develop smart economy, health industry and other new industries and new formats, and create a digital economy innovation and development pilot zone.
Article 3 Improve the development level of the education and medical industry
Explore the establishment of a medical personnel training and hospital accreditation standard system that is in line with international standards, relax restrictions on overseas doctors to practice in the mainland, and try first international cutting-edge medical technologies.


Article 7 Relaxation of access to international new drugs:
Allow designated medical institutions operating in 9 cities in the Guangdong-Hong Kong-Macao Greater Bay Area (Hong Kong and Macao medical and health service providers set up medical institutions in 9 cities in the Pearl River Delta in the form of sole proprietorship, joint venture or cooperation, etc.) to use urgently clinically needed medical institutions that have been listed in Hong Kong and Macao drug.
Article 4 Promote the facilitation of visas for foreign talents:
1. Support Shenzhen to explore and formulate standards for identifying foreign "high-tech and top-notch" talents, and apply for R visas for qualified foreigners.
12. Grant Shenzhen foreign high-end talents a confirmation letter authorization to explore and optimize the approval process for foreigners' work permits and work-type residence permits in China.
13. Provide entry and exit convenience for foreign high-level talents investing in entrepreneurship, giving lectures and exchanges, and economic and trade activities.
Article 5 Explore the convenience of residence for high-level foreign talents and the occupation list system for talents in short supply
Provide permanent residence convenience for foreign high-level talents. Formulate a list of occupations for talents in short supply, simplify the procedures for foreign talents to work in China, and improve the convenience of foreign talents to work in China.
Article 6 Implement a highly convenient practice system for overseas professionals
Provide Shenzhen with the authority to formulate overseas professional management regulations under the guidance of relevant departments, clarify professional conditions, business scope, etc., and allow financial, taxation, construction, planning and other professionals with overseas international professional qualifications to provide professional services in Shenzhen in accordance with relevant regulations . Relax restrictions on foreign personnel (excluding medical and health personnel) participating in various vocational qualification examinations.
Article 8 Explore and improve the cross-border connection mechanism of medical services:
Explore the establishment of a medical personnel training and hospital accreditation standard system that is in line with international standards.
(1) Building a collaborative innovation community in the Greater Bay Area
Article 9 Innovating the system and mechanism of Shenzhen-Hong Kong-Macao scientific and technological cooperation
Cooperate with Hong Kong to apply for a pilot reform of the drug bottle review system. Take the lead in promoting the cross-border use of medical data and blood and other biological samples required by scientific research projects in universities and scientific research institutions in Shenzhen, Hong Kong and Macao, and explore the mechanism for rapid customs clearance of samples and reagents.
(2) Promote the simultaneous leap of technological innovation capabilities
Article 10 Build a world-class major scientific and technological infrastructure cluster
Unite the superior scientific research forces of the Greater Bay Area to build a number of major scientific and technological infrastructures, and create a world-class major scientific and technological infrastructure cluster that is clustered in spatial distribution and connected in research directions.
Article 11 Jointly build world-class scientific research institutions
Promote the construction of important platforms such as the Shenzhen-Hong Kong International Multi-center New Drug Clinical Trial Base, the National Bio-innovative Drug Technology Review Institution, and the Clinical Application New Technology Trial Base.
(3) Create a first-class technological innovation environment
Article 12 Expanding the opening up of science and technology fields
Relying on the Shenzhen National Gene Bank to initiate the establishment of the "Belt and Road" Life Science and Technology Promotion Alliance.

Article 13 Promote the leapfrog development of life economy:
Cooperate with key universities and scientific research institutions in Hong Kong and Macau to jointly launch a major scientific plan in the field of life and health, promote the construction of a national bio-industry base with high standards, focus on the layout of the high-end biomedical industry chain and innovation chain, and build a national life and health industry innovation demonstration zone. Establish a multi-level basic support and common technology platform, make breakthroughs in key drug research and development technologies, improve the innovation capabilities of original drugs, first generic drugs and new preparations, and accelerate the construction of an international industrial belt for biomedicine in the Guangdong-Hong Kong-Macao Greater Bay Area. Build a collaborative innovation system for medical devices and vigorously develop high-end medical devices. Focus on the development of precision medicine, provide genetic testing technology and accelerate the clinical application of personalized medicine.
Article 14 Promote the leapfrog development of the marine economy in the Greater Bay Area
Article 15 Constructing a Shenzhen-Hong Kong-Macao Marine Technology Cooperative Innovation System
Actively build a land-sea big data center in the Guangdong-Hong Kong-Macao Greater Bay Area.
Article 16 Intensify the medical and health exchanges and cooperation between Guangdong, Hong Kong and Macao
Attract Hong Kong and Macao medical and health service providers to set up medical institutions in Shenzhen, explore the establishment of regional medical consortia, regional medical centers and specialist alliances in the Guangdong-Hong Kong-Macao Greater Bay Area, establish a mechanism for the prevention and control of infectious diseases in the Guangdong-Hong Kong-Macao Greater Bay Area, and promote the Guangdong-Hong Kong-Macao Greater Bay Area District medical and health information sharing platform construction. Establish a training and certification system for specialist physicians in line with international standards.
Article 17 Promote the innovative development of traditional Chinese medicine in Guangdong, Hong Kong and Macao
Deepen the cooperation with Hong Kong and Macao Chinese medicine testing institutions to jointly establish internationally recognized quality standards for Chinese medicine products. Promote the optimization of the approval process for Hong Kong and Macao Chinese medicine products entering the Mainland. Cooperate with a number of key universities in Hong Kong and Macau to carry out innovative research on modern Chinese medicine. Accelerate the construction of a traditional Chinese medicine research and production integration innovation center in the Guangdong-Hong Kong-Macao Greater Bay Area, and build a comprehensive innovation platform that concentrates drug product research and development, technological innovation, achievement transformation, standard upgrades, industrial services, and application demonstrations.
Article 18 Strengthen cooperation between Shenzhen, Hong Kong and Macao on food and drug safety:
Article 19 Create an innovative industrial cluster that leads the world
Actively create a national biomedical innovation policy experimental zone, strive to establish a national drug review center and a medical device review center Guangdong, Hong Kong and Macao branch, provide "one-stop" services for drug and medical device marketing approval and registration, and allow Hong Kong to self-register for those who have not yet been registered in China International new drugs and new medical devices are used in the cooperation zone to expand the use and clinical trials, and comprehensively promote precision medicine, digital life and other application demonstrations.
Article 20: First try to build a national offshore innovation zone
Strive to apply bonded policies in the Shenzhen area, exempt from compulsory product certification for the entry of scientific and technological research and development equipment, and implement catalog filing system management for samples, reagents, consumables and other materials needed for scientific research and production to facilitate customs clearance.
Article 21 Fully promote the construction of major scientific and technological installations
Build a batch of major scientific and technological infrastructure, and strive to introduce a batch of scarce national facilities that have been included in the major national plans.
Article 22 Vigorously promote basic research work
Vigorously support the construction of major research institutions and form strategic support for source innovation.
Article 1:Accelerate the high-quality development of Shenzhen's biomedical industry, and focus on improving the original innovation capabilities of the industry and the transformation of results.
Article 2:Applicable to institutions that are registered in Shenzhen and have independent legal personality to engage in drug and medical device research and development.
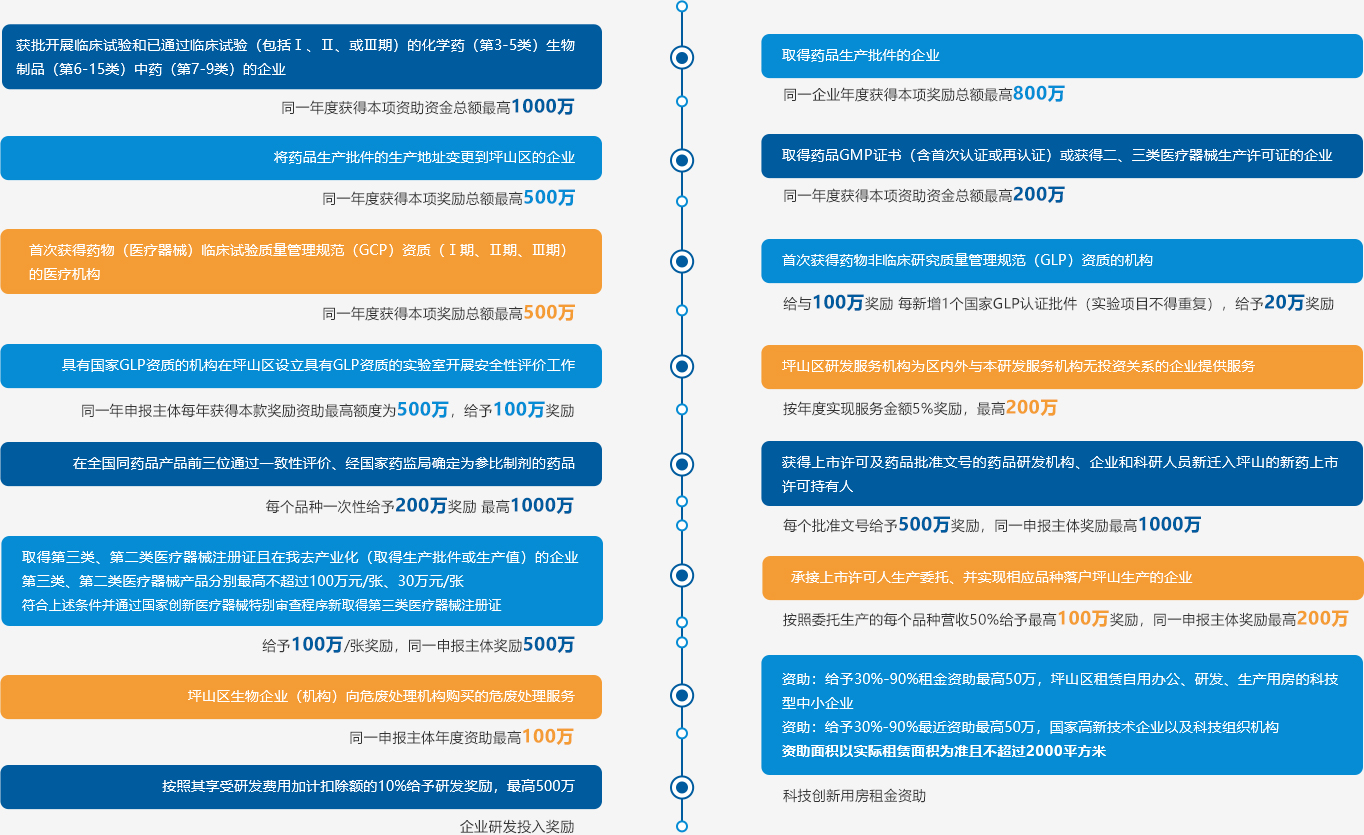
Article 3
![]()
![]()
![]()
Article 4
For the first three drugs that have passed the consistency evaluation of generic drugs in the country, 20% of the actual cost of consistency evaluation will be funded, and the maximum amount will not exceed 5 million yuan; the maximum annual funding of a single enterprise will not exceed 10 million yuan.
Article 5
The second and third categories of medical device products that have obtained the medical device registration certificate will be funded at 40% of the actual investment in research and development costs. The second and third categories of medical device products will not exceed 3 million and 5 million yuan respectively. The maximum annual funding for a single enterprise is not More than 10 million yuan.
Article 6
For biomedical companies in our city that undertake production in accordance with the MAH and medical device registrant system, they will be subsidized at 20% of the actual input costs, with a maximum of 15 million yuan for each product, and a maximum of 30 million yuan for a single company per year.
Article 7
For medicines and medical devices certified by international authorities such as the World Health Organization (WHO), they will be funded according to audited expenses, up to a maximum of 10 million yuan. For drugs that have been marketed overseas through international joint clinical research, 40% of the actual investment costs will be funded, and the maximum will not exceed 10 million yuan.
Article 8
Institutions that comply with the National Drug Clinical Trial Quality Management Regulations (GCP) or have obtained internationally renowned certification will be funded according to different percentages of the total project investment, up to a maximum of 5 million yuan.
Article 9
Gather the world's top scientific teams in the fields of synthetic biology, brain science, precision medical imaging and other fields to accelerate the construction of major scientific and technological infrastructures. Project construction and operating funds are allocated from the investment of the municipal government.
Article 10
Support the breakthroughs in new drugs and high-end medical devices that are expected to solve major clinical needs and market needs, and provide full funding for their preliminary research, up to a maximum of 30 million yuan; focus on common key technologies such as high-end medical devices and innovative drug research and development, and solve For the problem of "stuck neck", 40% of the total investment of the project will be funded in stages, and the maximum will not exceed 300 million yuan.
Article 11
Accelerate the introduction of industry-leading CRO C(D)MO drug toxicology research and other biomedical industry application and service basic platforms, and 40% of the total project investment will be funded, and the maximum will not exceed 50 million yuan.
Article 12
Accelerate the construction of third-party testing service platforms such as Shenzhen Generic Drug Quality and Efficacy Consistency Evaluation Technology and Standard Research Platform, and provide annual support based on 40% of the actual project's total investment, with a maximum of 30 million yuan.
Article 13
Encourage medical institutions to cooperate with universities and scientific research institutions in the Guangdong-Hong Kong-Macao Greater Bay Area to establish a Shenzhen-Hong Kong International Clinical Medicine Research Center in the Shenzhen-Hong Kong Science and Technology Innovation Cooperation Zone to carry out new technology development and applied research.
Article 14
Actively strive to establish the Guangdong-Hong Kong-Macao branch of the Center for Drug and Medical Device Evaluation (CDE) to promote the innovative development of the biomedical industry.
Article 15
Support the construction of public service platforms such as drug screening, drug evaluation, and GLP laboratories in the park or in the park, and subsidize 40% of the total investment, up to a maximum of 10 million yuan.
Article 16
Major biomedical industry projects that have an investment of more than 2 billion yuan or can fill the gaps in the industrial chain and have particularly outstanding economic and social benefits will be given key support after being reviewed and approved by the municipal government.
Article 17
Eligible biomedical institutions and enterprises will be subsidized according to 50% of their actual premiums. The maximum amount of individual insurance policies shall not exceed 500,000 yuan, and the maximum annual subsidy of individual enterprises shall not exceed 5 million yuan.